Treatment of peri-implant mucositis
Treatment of peri-implant mucositis
Peri-implant mucositis is mucosal inflammation in the absence of marginal peri-implant bone loss beyond crestal bone level changes resulting from initial bone remodelling.10, 123 Clinical signs include redness, swelling, bleeding on gentle probing at more than one site around the implant, and suppuration (see figure: Peri-implant mucositis).
The goals of treatment are to achieve bleeding on probing at ≤1 site (bleeding should not be profuse) and absence of suppuration around the affected implant at 2-3 months after treatment.10
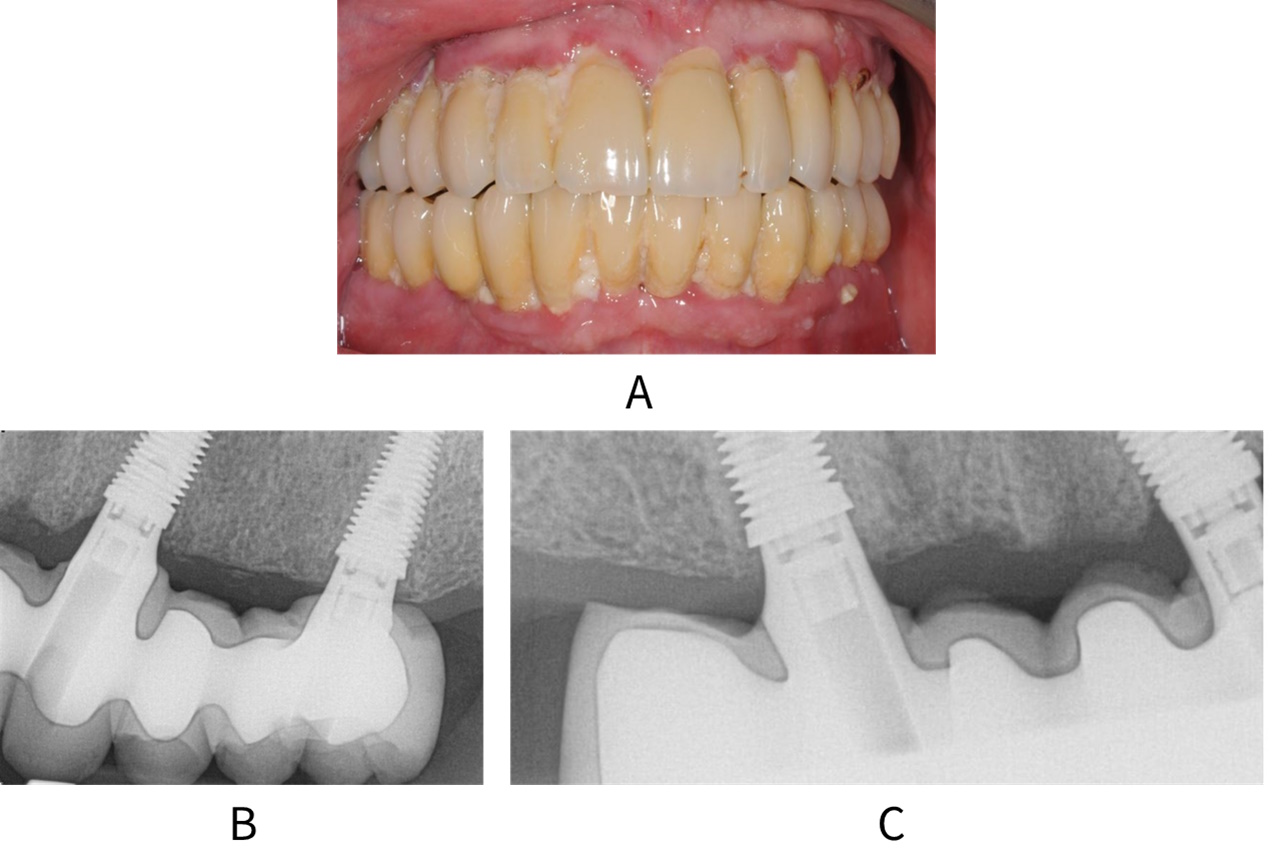
A: Patient with full upper and lower arch restorations and peri-implant mucositis. Radiographs B and C show no bone loss around the implants
Key recommendations
For patients with peri-implant mucositis, the routine use of adjunctive or alternative measures to professional mechanical plaque removal is not recommended.
(Conditional recommendation; low certainty evidence)
The routine use of local or systemic antibiotics for the treatment of peri-implant mucositis in primary care is not recommended.
(Conditional recommendation; low certainty evidence)
A recent consensus statement from the World Dental Federation noted that conventional non-surgical professional mechanical plaque removal (PMPR), in conjunction with oral hygiene reinforcement, is the standard treatment for peri-implant mucositis.124 Evidence suggests that specific alternative or adjunctive therapies, such as antiseptics, antibiotics or treatment using lasers, do not significantly improve clinical outcomes when compared with PMPR alone.125-128 The certainty of the evidence is considered to be low due to risk of bias and inconsistency.
In addition, the EFP Prevention and treatment of peri-implant diseases guideline10 recommends PMPR in combination with oral hygiene re-enforcement for the management of peri-implant mucositis. PMPR can be performed with ultrasonic or air polishing devices or with hand instruments. Short term use of patient-administered oral antiseptic rinses can be considered. The guideline does not recommend combining modes of PMPR or using lasers in combination with conventional PMPR. It also does not recommend the use of systemic or locally administered antibiotics as adjuncts to PMPR. It suggests not to use professionally administered local agents (e.g. antiseptics) or photodynamic therapy as adjuncts to PMPR.
Further details on the development of the recommendations in this guidance can be found in Methodology.
If soft tissue inflammation is present:
Exclude the presence of peri-implantitis by carrying out a radiographic examination of the implant to assess peri-implant bone levels compared with the baseline radiograph.
- Periapical radiographs, taken using the long cone paralleling technique, are appropriate for assessing peri-implant bone levels.
- If bone loss is observed, refer to Treatment of peri-implantitis.
Check the restoration contour to ensure that patient performed oral hygiene is possible.
- If the restoration does not allow access for home care, consider whether it is possible to recontour or replace the restoration to allow adequate oral hygiene.
Check for the presence of retained cement around the restoration.
Inform the patient of their diagnosis and any associated factors that increase their risk of disease.
Provide personalised oral hygiene advice and instruction to assist and encourage the patient to improve their oral hygiene skills as well as their understanding of the value of good self-care routines (see Oral hygiene).
Encourage the use of implant-specific oral hygiene aids such as implant floss and interdental brushes.
Where applicable, give smoking cessation advice and support (see Smoking cessation).
Carry out professional mechanical plaque removal of the restoration and implant surface.
- Remove supramucosal and submucosal plaque and calculus and any retained cement using an appropriate method. Local anaesthesia may be required.
Arrange a review appointment after 1-2 months to assess the outcome of treatment.
If inflammation is still present, repeat non-surgical treatment and:
- Arrange review at 3 months;
- Consider further radiographic assessment at 3-6 months to review bone levels.
Ensure regular, risk-based recall for maintenance of the implants and their restorations is arranged.